Making a Rechargable Battery (NiOOH-Zn)
For my second year project course (the same one I later TA'd) I created a rechargable battery from scratch. Our professor of the class stood up at the front of the class, wrote the following line on the board, said we had 4 months to do so and walked out:
Make a battery.
Half the class laughed, they said they were going to make a lemon battery. We had 4 months to do so, what a joke!
I did not feel that way, I wanted to produce something awesome. I wanted to make a rechargable battery. My group and I started looking up easy to build DIY rechargable batteries and came across the Ni-Zn batteries. A fairly unusual battery and largely not used.
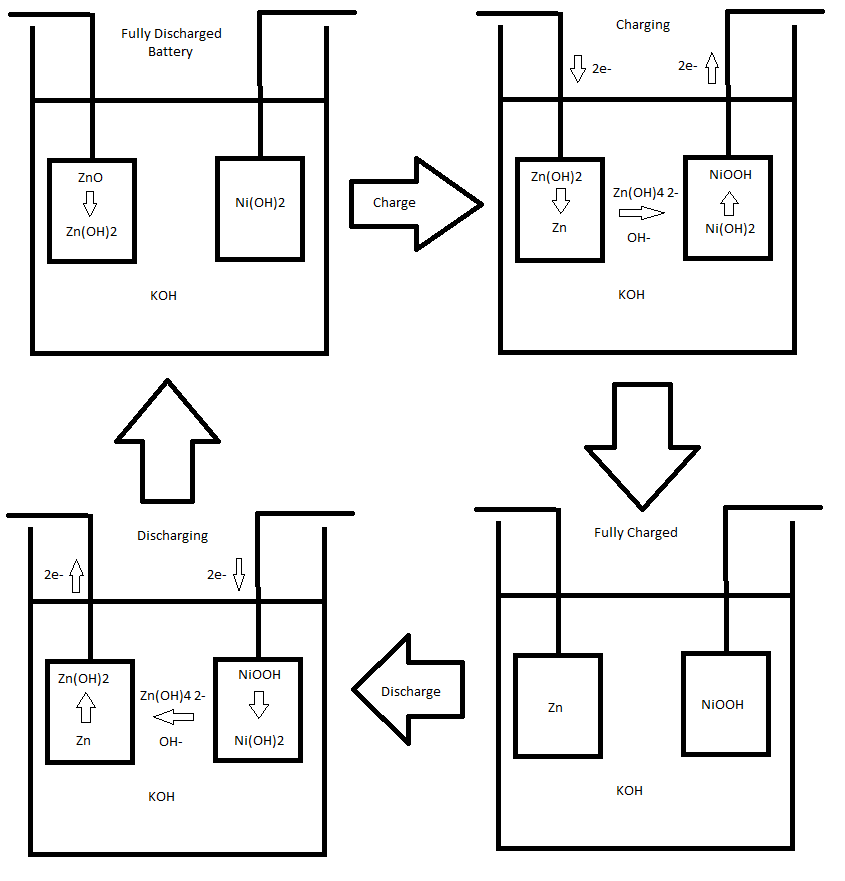
How the battery operates is illustrated below.
The overall reaction of the cell is the following:
ZnO + Ni(OH)2 + H2O = 2NiOOH + Zn
What we needed however was a nickel hydroxide electrode and a sintered zinc oxide electrode. Both of which we had no idea how to produce. So after doing some research we came up with a few procedures.
Synthesis of Nickel Hydroxide Electrode
The goal of this procedure was to chemically impregnate a Ni foam with Nickel hydroxide. We want to crystallize nickel hydroxide into the pores of the foam such that the foam can act as a current collector.
Nitric acid was added to fine nickel powder to produce nickel nitrate. In a separate beaker, potassium hydroxide was dissolved in water.
Nickel Hydroxide on the top and Nickel Oxy-Hydroxide on the bottom
The Ni foam was taken with tweezers and dunked into the nickel nitrate solution, filling all of its pores with the solution. It was then abruptly dunked into the basic potassium hydroxide solution to turn the nitrated nickel into nickel hydroxide. The nickel hydroxide precipitates into a crystalline solid and since its already in the pores of the foam, it stays inside trapped. For a close up macro view of what the crystalline nickel hydroxide looks like inside the pores of the foam, see below. Beautiful isn't it? I bet you thought that was water when you clicked the thumbnail!
Synthesis of Sintered Zinc Oxide Electrode
What is sintering anyways? Well what if you have a powdered ceramic material and you want to fuse the powder grains together? This is sintering. By compressing the material and applying heat, you can push the grains together such that they stick. Most ceramics you see are sintered using heat and pressure.
So we did the same, we applied around 40 MPa of force near 1500 K to a bunch of zinc oxide powder and created a compacted puck
The puck had a very smooth non-porous outer surface.
Battery!!
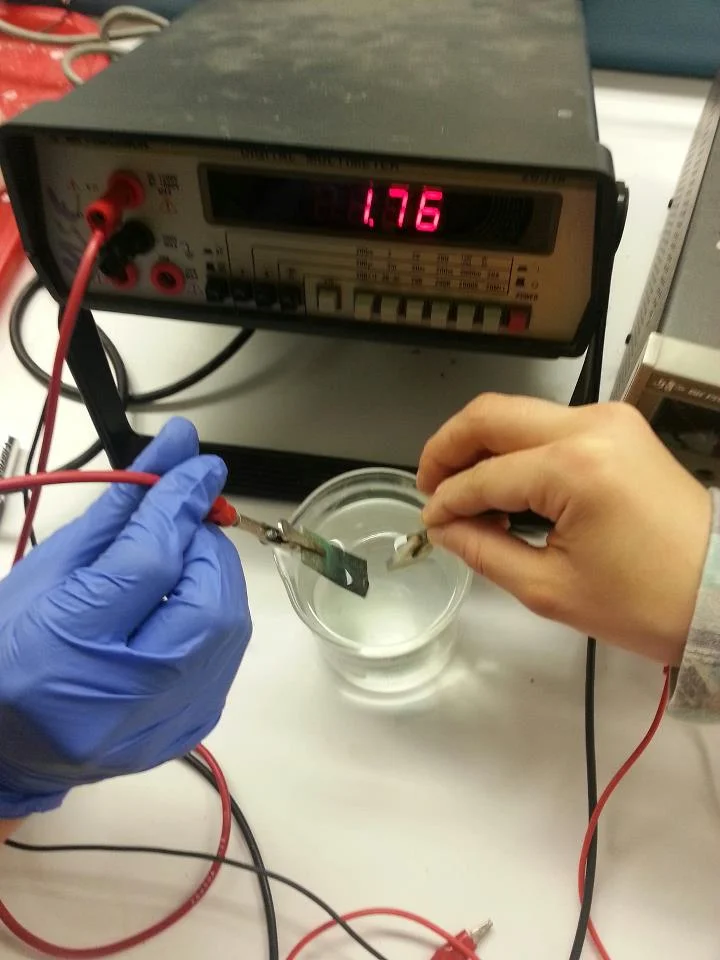
Next we attached aligator clips to each electrode, dunked it in caustic potassium hydroxide electrolyte and turned it on! We measured the voltage, and bam! 0V.
It did not work.
Bummer right?
We looked over at the zinc electrode and it was sizzling in surface cracks, but not anywhere else. Shoot, was the outside surface passivating the reaction? It was preventing diffusion of electrolyte into the interior of the ceramic.
So we cracked it open on that hunch. Into two pieces. Pretty bold considering it took us all semester to make it.
And voila.
Beautiful! We got it working! We charged it all the way until we started producing Zinc metal since zinc oxide gets reduced into zinc. Then we tried discharging it using a lightbulb.
Below is what the before and after looks like on the ZnO electrode.
You can clearly see a grey zinc color on the top electrode! We did it!
This was so exciting at the time.