Laser Powered CO2 Scrubbing Weather Balloons
You heard that right! This was for my final Engineering & Society capstone senior project. I was enrolled in the Engineering & Society program which is a program that in addition to your major (Materials Science & Engineering in my case) you take additional core courses in environmental, ethics, sustainability and communication. The intent of the Engineering & Society program was to create well-rounded engineers with a holistic approach to problem solving.
For our project, we partnered with a local early stage startup (alumni to our program). He had an idea of placing weather balloons powered by a beam of microwave beams high above the cities which could deliver telecommunications to the city below. He was curious how feasible placing a CO2 scrubber on the balloon would be. He wanted a feasability study done on the pros and cons. I believe the goal of the project was to add a sustainability arm to his idea. Shooting microwave beams above a densely populated area is not well received by the masses. But saying part of the reason is to reverse global warming? Now we're in business.
I was the technical lead for this project, and I came up with a few different methods of scrubbing CO2. Biological scrubbers (algae based), chemical scrubbers (monoethylamine based) and physical/thermodynamic scrubbers (cryogenic liquefaction).
Below is an overview of my liquefaction technique.
Overview
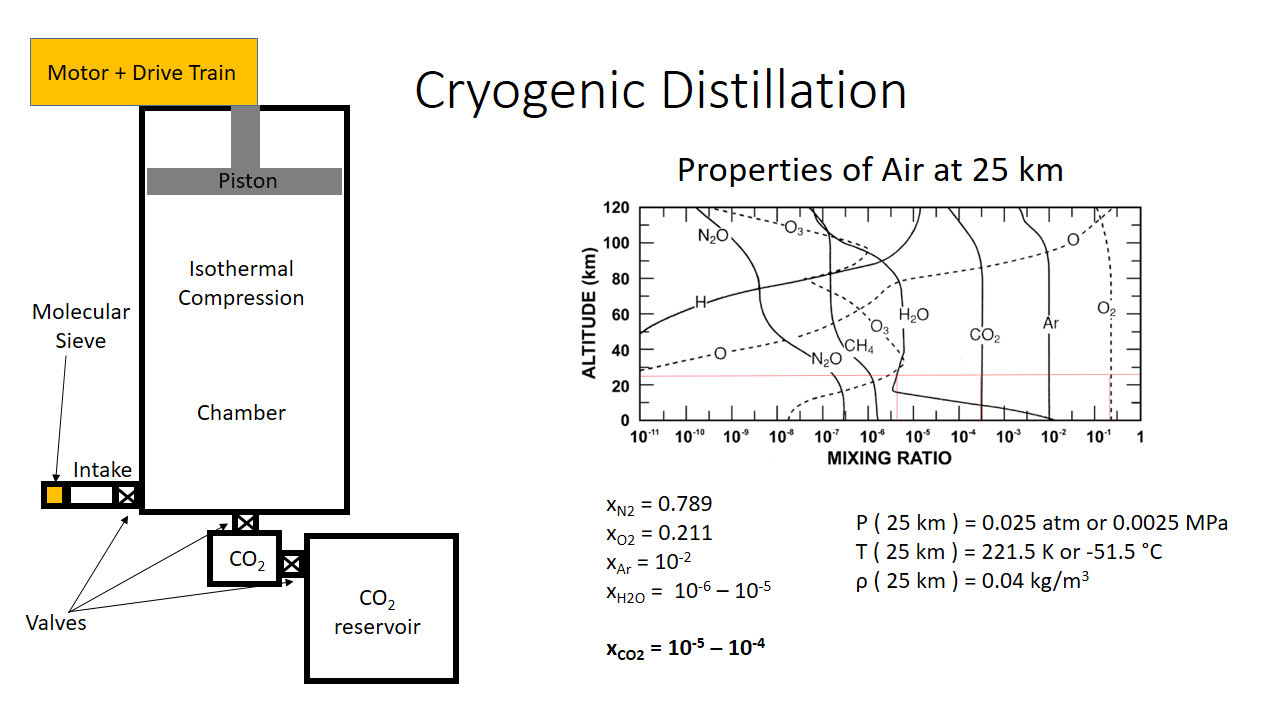
Above is an overview of the multistage device. The air is first intaken through a molecular sieve (the sieve dries the air from moisture). It then passes through a valve and enters a main chamber, then a secondary chamber is a transient CO2 container, then finally a CO2 reservoir. The red lines above on the properties of air at 25 km chart shows what the mixing ratio of gases is at 25 km in altitude. There are additional thermodynamic properties at this altitude listed above as well.
In addition to this, it should be noted the CO2 reservoir is already at low pressure. It is sealed under vacuum at 10^-5 atm . I will explain this choice later.
Step 1
Green valves indicate they are open, whereas red valves indicate they are closed. The red dot on the phase diagrams above tell us where we exist at the pressures and temperatures listed. According to the water P-T phase diagram we are well within a solid phase which is why we must adsorb CO2 onto the molecular sieves in the intake. CO2 on the otherhand is very much a gas. We then equilibrate the main chamber and the transient CO2 container by opening the green valves above.
Step 2
We then close the intake valve and compress the main chamber with the piston. As seen in the above phase diagram we quickly shift the equilibrium phase of CO2 from gas to liquid. We assume isothermal compression (that is, compression where temperature does not change) because the exterior of the chamber is fixed at -50 Celcius, it is 25 km in altitude after all. For isothermal compression is also trivial to calculate the work required for the stroke as well :)
With the CO2 liquified, we note that volumes of the containers were chosen so the above will hold. See the report at the end of this post for a detailed schematic. With liquid overflowing the transient CO2 chamber, we move onto the next step.
Step 3
We then close the transient CO2 valve, and open the intake valve to allow the main chamber to be equilibrated once more.
Step 4
Notice the initial pressure in the final CO2 reservoir (P4) is starting at 10^-5 atm. This was deliberate. The high vacuum container needs to be pre-pumped on the ground before it is attached to the balloon. It is apparent for this step when we see the transient CO2 container will equilibrate with the CO2 reservoir which is at lower pressure. The lower pressure will cause an expansion of the liquid CO2 and turn it back into a gas which now occupies both the CO2 reservoir and the transient CO2 container. With the container having a significantly larger volume as the transient CO2 container, the majority of the CO2 will indeed be in the reservoir.
Step 5
With the reservoir now filled with CO2, the valve between the transient container and the reservoir is closed and the valve between the transient container and the main chamber is opened for equilibration. The system is ready for another cycle. The reservoir will be considered "full" when it reaches 6 atm. It is at this point when there is no more driving force between the transient container and the reservoir container.
A whopping 917 MJ of energy is required to sequester 1 kg of CO2! Damn!
Yeah that's quite a lot. Okay now for the real interesting bit. Is it cheaper to do it at 25 km or at sea level?
We know the thermodynamics is different at 25 km in altitude due to the T and P effects.
There are indeed some cost savings! ~70 MJ of energy worth which is pretty small to be honest.
Anyways I hoped you enjoyed my take on chemical engineering. Although I do have an extensive background in thermodynamics, my experiences are mostly with bulk and nanoscale solid state (predominantly binary solid solutions) thermodynamics. My gas thermodynamics is limited, but most of this work is ideal anyways.